Chemical shift, spin-spin interaction and J-coupling
Chemical shift corresponds to a change in the resonance frequency of the nuclei within the molecules, in function of their chemical bonds. The presence of an electron cloud constitutes an electronic shield that slightly lowers the B0 magnetic field to which the nucleus would normally be subjected. The resonance frequency difference is expressed as parts per million or ppm, a value that is independent of the amplitude of the magnetic field. The value of the chemical shift thus provides information about the molecular group carrying the hydrogen nuclei.
Chemical shift value
For a given molecule, the chemical shift in ppm versus the reference molecule is defined by the relationship:
With:
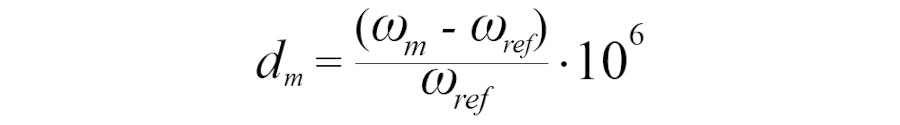
- ωm: resonance frequency of the studied molecule
- ωref: resonance frequency of the reference molecule
- dm: chemical shift value in ppm.
In chemical shift, the difference in frequency compared to the reference molecule is proportional to the amplitude of the B0 magnetic field.
Interaction between the atomic nuclei of neighboring chemical groups translates as each peak breaking down into a complex peak (doublet, triplet, multiplet): this is spin-spin coupling. The spacing between these peaks has a fixed frequency value (Hz), called the J-coupling constant, independent of magnetic field amplitude.
A spectrum is represented:
- as an abscissa: metabolite position according to chemical shift. Tetramethylsilane is used as the axis origin reference, a molecule used in in-vitro NMR, which is conventionally at 0 ppm. This axis is orientated from right to left.
- as an ordinate: peak amplitude.
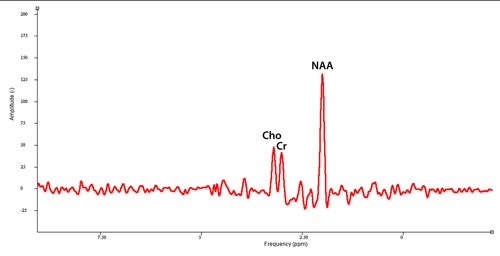
The area below the peak curve will basically be determined by metabolite concentration. The width of the peak is inversely proportionate to T2* relaxation time.
